In this video recorded for the ARVO 2020 Annual Meeting, Dr. Stephen R. Russell presents the 12-month results of a phase 1b/2 trial of sepofarsen. Following the results presentation, Dr. Russell and Ben Shaberman (Foundation Fighting Blindness) discuss the results, the potential and future of RNA therapies for inherited retinal diseases (IRDs). The Q&A starts at 09:29.
Results of a phase 1b/2 trial of intravitreal (IVT) sepofarsen (QR-110) antisense oligonucleotide in Leber congenital amaurosis 10 (LCA10) due to p.Cys998X mutation in the CEP290 gene
Published on at ARVO
Presenter(s)
Stephen R. Russell1, Arlene V. Drack1, Artur V. Cideciyan2, Samuel G. Jacobson2, Bart P. Leroy3,4, Wanda L. Pfeifer1, Alina V. Dumitrescu1, Alexandra V. Garafalo2, Allen C. Ho5, Caroline Van Cauwenbergh3, Julie De Zaeytijd3, Aniz Girach6, Wil den Hollander6, Michael R. Schwartz6, David M. Rodman6
1 Department of Ophthalmology and Visual Sciences, Carver College of Medicine, University of Iowa, Iowa City, IA, USA; 2 Scheie Eye Institute, Department of Ophthalmology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 3 Department of Ophthalmology & Center for Medical Genetics, Ghent University Hospital & Ghent University, Ghent, Belgium; 4 Ophthalmic Genetics & Visual Electrophysiology, Division of Ophthalmology, The Children’s Hospital of Philadelphia, PA, USA; 5 Wills Eye Hospital, Thomas Jefferson University, Philadelphia, PA, USA; 6 ProQR Therapeutics, Leiden, the Netherlands.
Description
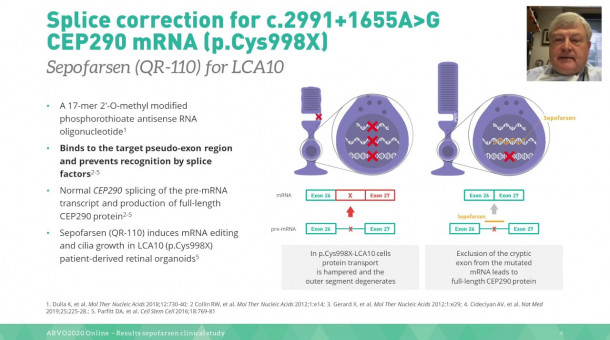
In a phase 1b/2 clinical trial, 11 Leber congenital amaurosis 10 (LCA10) patients received intravitreal sepofarsen. The goal was to evaluate safety and change in various ophthalmic endpoints from baseline to month 12. Sepofarsen had a manageable safety profile and showed improvement in mean visual acuity, and full-field stimulus testing.

Video-recorded presentation
Sepofarsen Phase 1b/2 trial results for LCA10
ARVO 2020 presentation and Q&A by Dr. Stephen R. Russell
The video-recorded results presentation is also available on ARVO Learn, ARVO’s online learning platform.
Related publications
-
-
Full field stimulus testing (FST) to assess sepofarsen patient response in Leber congenital amaurosis type 10
Date:
Author(s): Ho A, Cideciyan AV, et al.
ePoster -
Results of a phase 1b/2 trial of intravitreal (IVT) sepofarsen (QR-110) antisense oligonucleotide in Leber congenital amaurosis 10 (LCA10) due to p.Cys998X mutation in the CEP290 gene
Date:
Author(s): Russell SR, Drack AV, et al.
ePoster